
The vascular surgery research laboratory has been extensively involved in four main areas of vascular biology research: 1) evaluating mechanisms responsible for prosthetic graft failure 2) prevention of intimal hyperplasia in vein grafts 3) developing novel biomaterial surfaces and 4) investigating the role of neuropeptides in diabetic wound-healing.
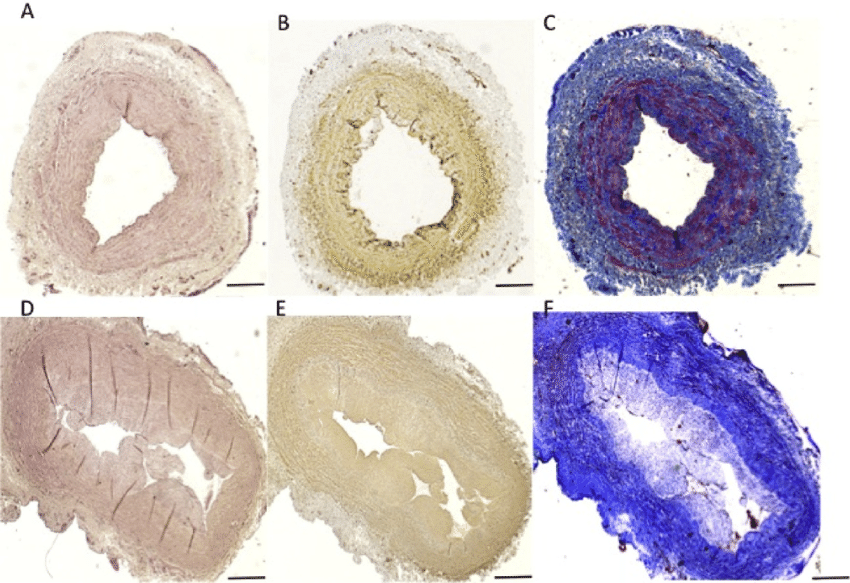
Anastomotic intimal hyperplasia (AIH) is the most common cause of delayed prosthetic arterial graft failure, and delayed failure of vein grafts. As graft healing occurs, genes are either up- or down-regulated as compared to a quiescent arterial wall. We study altered gene expression that results in endothelial cell activation as well as cellular proliferation, migration and extracellular matrix production by smooth muscle cells, leading to AIH. Differential gene expression is assessed using various techniques such as, microarray analysis, qPCR immunohistochemistry, and laser capture microdissection (LCM), a relatively new technology developed by the National Institutes of Health which is available at Beth Israel Deaconess Medical Center. We have now established proficiency with LCM, a tool designed to isolate homogeneous populations of cells for genetic analysis. This technique allows direct microscopic visualization and isolation of selected cell populations from frozen tissue sections and permits selection of cells within a chosen area of tissue. The significance and unique aspect of this work is that our group is the first to identify specific genes that are altered following prosthetic arterial grafting and vein grafting in vivo and to examine their role in the cellular environment using various in vitro cell culture assays. This information is now being used to identify targets for RNA interference-mediated gene silencing. We have established our ability to silence RNA in cell culture and in the vein graft wall and are working to develop systems for RNA silencing that will be practical for intraoperative use. This research is funded by a R01 grant from the National Heart Lung and Blood Institute at the National Institutes of Health.

The other aspect of our research is to develop novel cardiovascular device surfaces for both peripheral and cardiac applications. Several novel surfaces have been developed and are currently being evaluated both in vitro and in vivo. We have designed and patented a novel, biocompatible Dacron vascular graft that has the following properties: 1) reduced porosity via sealing with a novel ionic urethane, 2) mechanical properties comparable or superior to the pre-coated Dacron substrate and 3) localized immobilization of select proteins to the graft surface. This composite graft has been characterized in vitro and will be assessed in vivo this year. Another project currently ongoing is to developing a novel small-diameter (4mm internal diameter) prosthetic vascular graft using nanofiber technology with surface antithrombin properties. In collaboration with the Division of Podiatry at the BIDMC, we are actively developing in vivo and in vitro models to study diabetic wound healing. Peripheral neuropathy and peripheral vascular disease are the major contributors to diabetic foot ulcers (DFU) and their failure to heal. Therefore, it is important to assess the individual and combined role of neuropathy and vascular disease and their intricate interplay that leads to DFU. To achieve this goal we have successfully developed an in vivo diabetic rabbit model that addresses the effects of neuropathy and ischemia on wound healing. Our in vitro studies using dermal vascular endothelial cells are specifically designed to monitor the role of neuropeptides in angiogenesis in a hyperglycemic environment.
The vascular surgery research laboratory is the lead laboratory for a T32 research training program from the National Institutes of Health. This program is designed to provide two years of intense basic research training in vascular surgery for future academic clinicians. The training program addresses the absence of adequate research training for vascular surgeons as it applies to specific areas of clinical disease. Trainees pursue a program of research activity supplemented with course work in research design, ethics, statistics and evaluation of published research in the areas of molecular and cell biology, biomechanics, coagulation and thrombosis and angiogenesis. Clinically relevant problems such as atherogenesis, intimal hyperplasia, prosthetic/host interactions and thrombosis are the main focus of these research projects. Trainees carry out their research projects under the guidance of a faculty advisor selected from 20 renowned vascular researchers based at four Harvard Medical School hospitals (Beth Israel Deaconess Medical Center, Brigham and Women’s, Children’s Hospital (Boston) and Joslin Diabetes Institute) and the Massachusetts Institute of Technology. Upon completion of the program, trainees are capable of independent research and possess the scientific and research background necessary to obtain peer-reviewed funding.